Nova Southeastern University Standard Operating Procedure for GCP Title: Essential Documents for a Clinical Trial Version # 1 S

Table 1 from Time-Sinks during the Planning Phase of Investigator Initiated Trials (IITs).Results of an Online Survey | Semantic Scholar
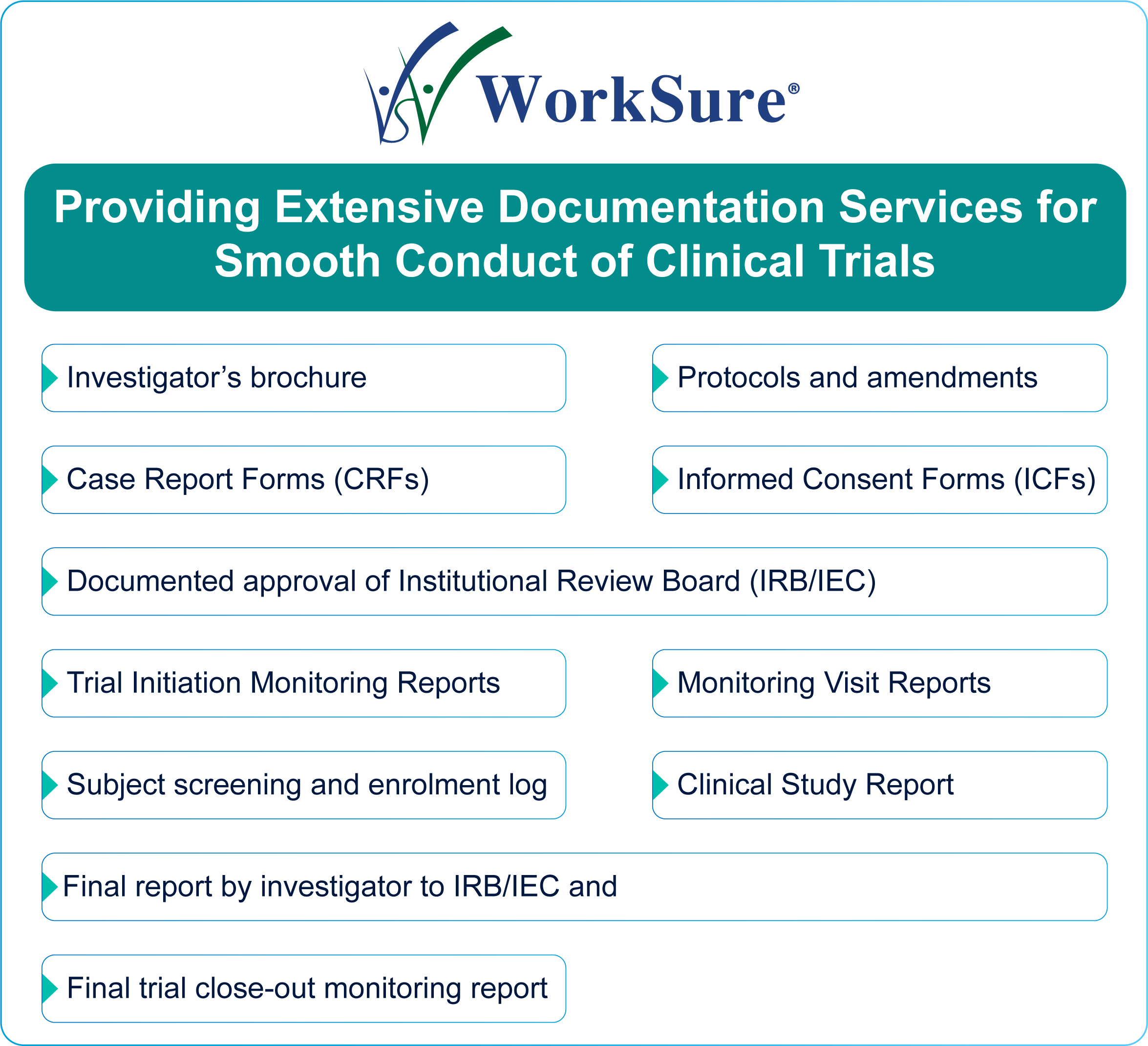
Role of Extensive Documentation in Smooth Conduct of Clinical Trials | WorkSure™ MedPharma Consultancy India Pvt. Ltd.

ICH GCP - 8. Essential documents for the conduct of a clinical trial: ICH E6 (R2) Good clinical practice















