
What is an IRB? And Why Should I Care? - DOCUMENT TRANSLATION, AUDIO TRANSCRIPTION, & PHONE INTERPRETATION
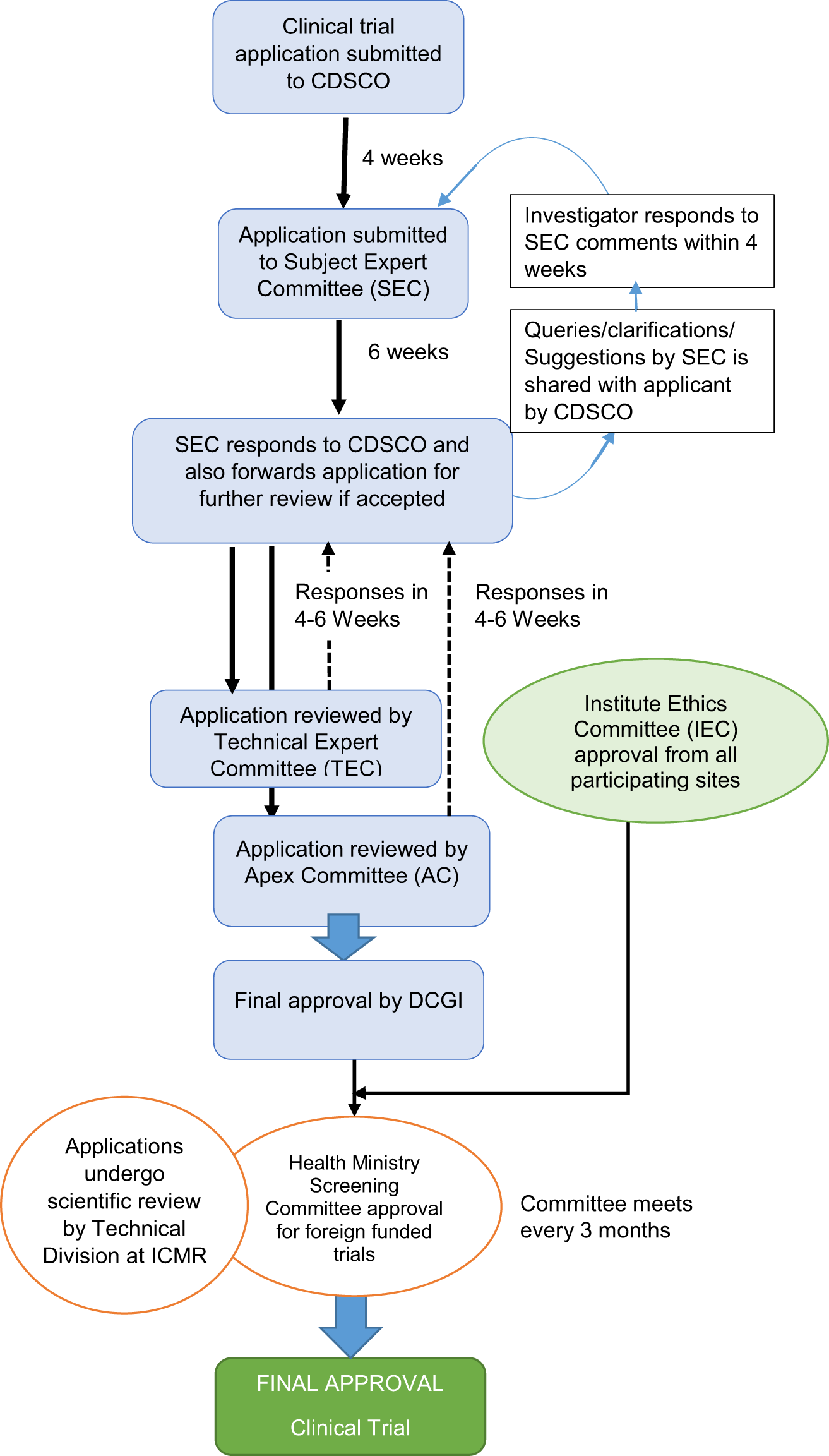
Issues, challenges, and the way forward in conducting clinical trials among neonates: investigators' perspective | Journal of Perinatology


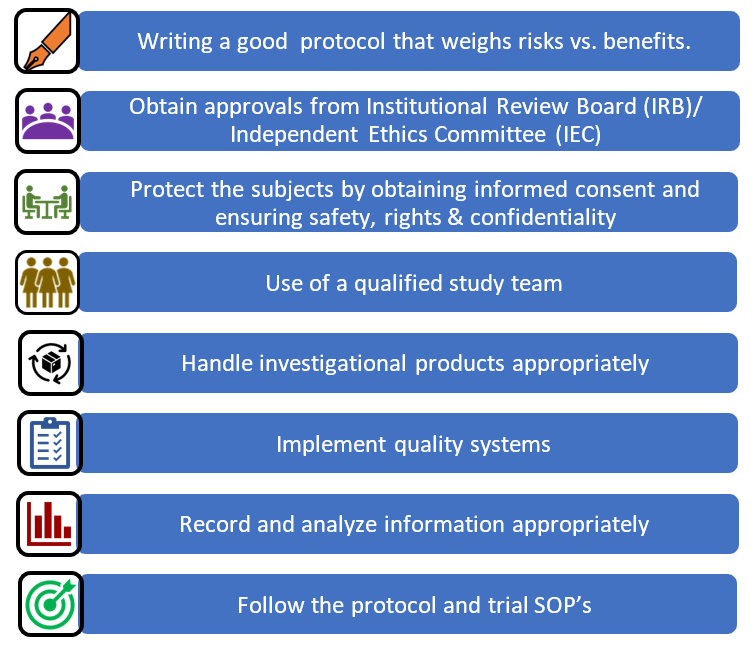



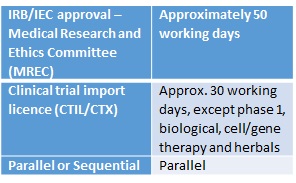


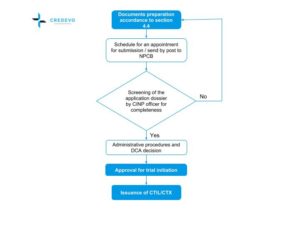






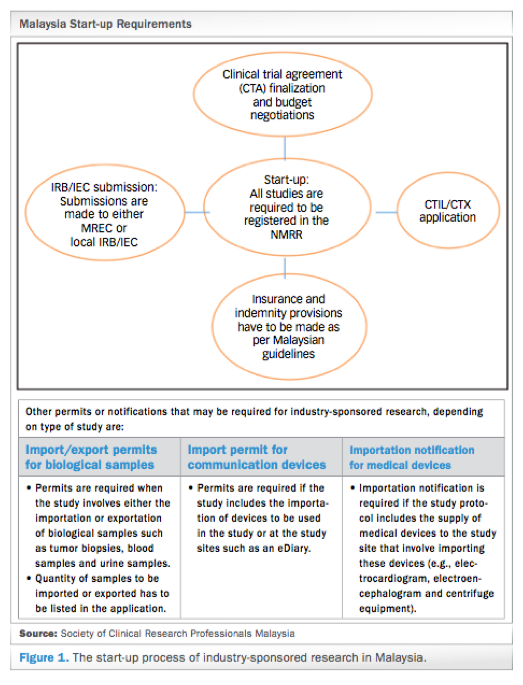




![PDF] Institutional Review Boards and Independent Ethics Committees | Semantic Scholar PDF] Institutional Review Boards and Independent Ethics Committees | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/057fccd15f43969114b301ea374eaaf0bcb9caa1/7-Table8.3-1.png)