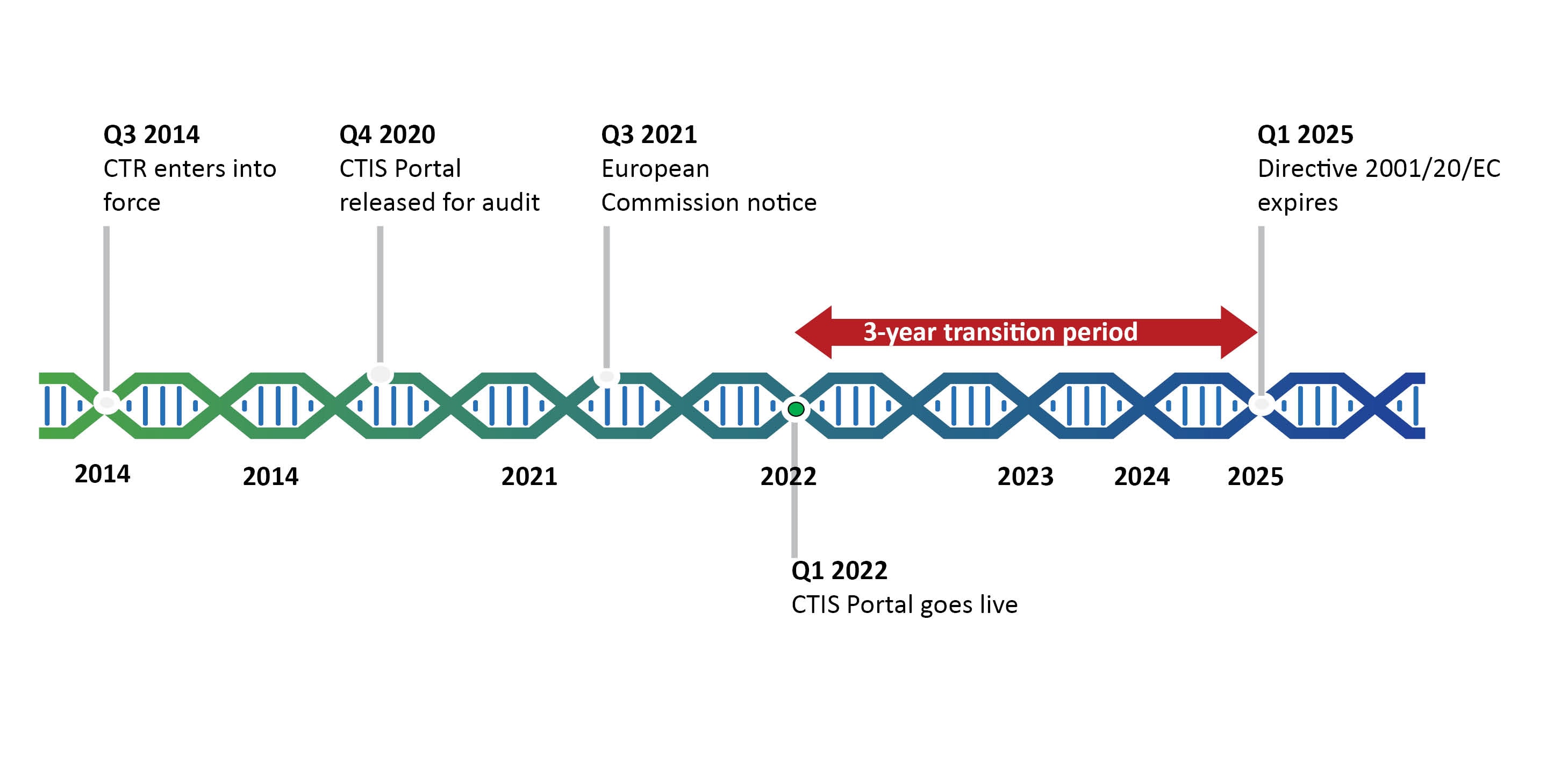
ADVANCED CLINICAL TRIALS THE CLINICAL TRIAL PROCESS: IMPENDING CHANGES IN THE REGULATORY FRAMEWORK - ADVANCED CLINICAL TRIALS

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library